Dr Nicki Grint
BVSc PhD DVA DipECVAA MRCVS
RCVS and European Specialist in Veterinary Anaesthesia
Background
Max was an 8-week-old cross bred puppy, weighing in at only 2.68kg when he was referred to the specialist centre where our anaesthesia consultant, Dr Nicki Grint was working. Max had been vomiting regularly since early that morning, and his referring vet had palpated a firm object in Max’s mid-abdomen. Their suspicions of a foreign body were confirmed after taking a conscious abdominal radiograph (figure 1); a radio-opaque object was apparent in the mid-ventral abdomen, looking suspiciously like a baby’s dummy. When questioned the owner reported that Max had developed a liking for chewing dummies, although had never eaten one before. That said the owner had recently found the hard plastic section of one such dummy that Max had taken a shine to, but not the softer teat section.
Max was referred for further investigations and treatment. When he arrived at the referral centre, a conscious abdominal ultrasound scan indicated that there was focal peritonitis surrounding the small intestinal segment containing the foreign body, but no free fluid. No other foreign bodies could be identified but an exploratory laparotomy was indicated. He was assessed as 5% dehydrated. Nicki requested acid base and electrolyte analysis of his blood as he had been persistently vomiting, but all of his parameters, including blood glucose were within normal limits. Whilst Max was being rehydrated with Hartmann’s intravenous fluid therapy, Nicki created his anaesthetic plan, taking into account his age, size, and proposed procedure.
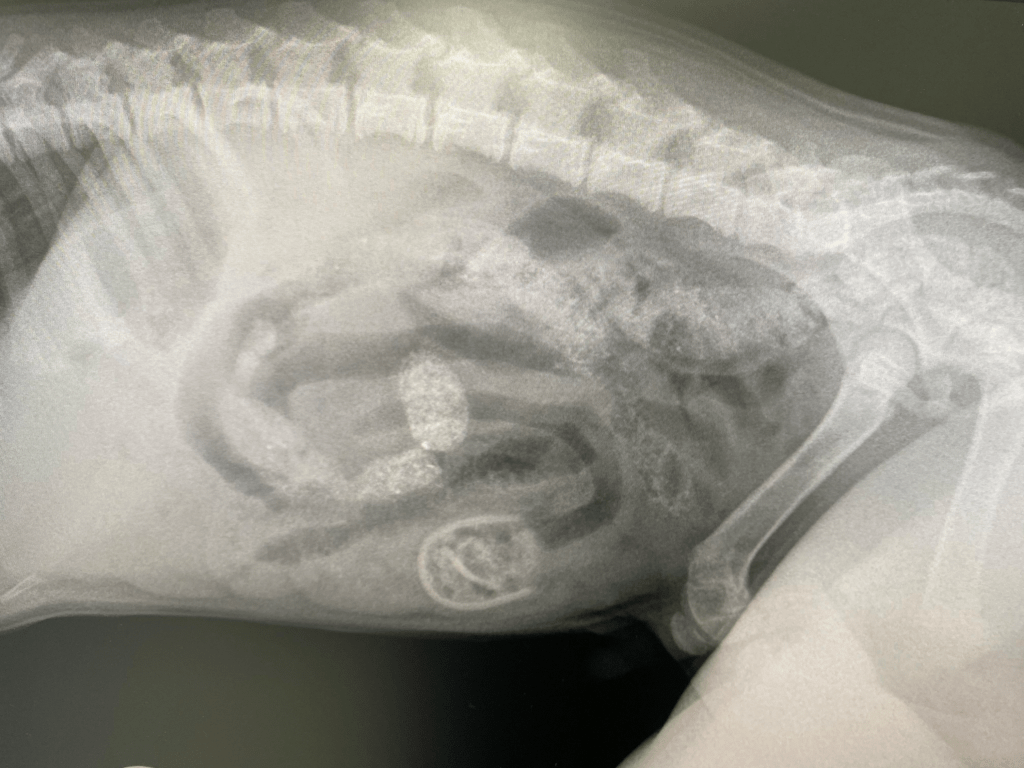
Figure 1: An oval, well-defined jejunal foreign body localised in the caudoventral part of the central abdomen. There is also evidence of secondary mechanical ileus.
Anaesthetic considerations
First and foremost in Nicki’s mind was Max’s age, as he was considered a paediatric patient at just 8 weeks old. Up until 12 weeks of age, several of the organ systems will be underdeveloped;
Respiratory: A paediatric patient will have a 2-3x increase in tissue oxygen demand and a higher respiratory rate. Their functional residual capacity is lower than an adult’s which means they will be more prone to desaturation. In relation to the oral cavity, the tongue is relatively large, yet the diameter of the airway narrow.
Cardiovascular system; The myocardium is very stiff in paediatric patients and so they cannot increase stroke volume if they need to increase cardiac output. Therefore they are heart rate dependent for maintaining their cardiac output. In general, it is best to avoid drugs which produce bradycardia in this age range of patients.
Hepatic physiology; The paediatric patient will have an immature microsomal enzyme system until they are 8-12 weeks old, which means they are slower to metabolise drugs. This could suggest that either lower doses of drugs are used, or with some drugs such as opioid analgesics instead of regimented dosing times (e.g. every 4 hours) that the drug administration is based on pain scoring, because the drugs may last a lot longer than usual. Glycogen storage is minimal and they have poor gluconeogenic ability which means these young animals are prone to hypoglycaemia.
Thermoregulation; Animals are born with very little subcutaneous fat, and together with their poor thermoregulatory capabilities and an immature control of vasomotor tone (i.e. they don’t have as much control of vasodilation and vasoconstriction) they are very prone to hypothermia. This will be compounded if they have prolonged pre-anaesthetic starvation, as metabolism generates body heat. As they are of a small size, they have a high surface area to volume ratio which means they are more prone to heat loss.
Pain and analgesia; Paediatric animals have a constantly developing physiology of many body systems until they reach maturity. Their central nervous system, including their ability to process pain is no exception. There is a lot of evidence that subjecting neonatal animals to pain during this time of development can be very detrimental to them, worsening their response to pain later in life.
Max’s small size was also taken into account; Nicki was aware that he would potentially become hypothermic during anaesthesia and so pre-warming with a forced warm air blower was started whilst Max was being rehydrated. During this time, appropriately sized anaesthetic equipment was also set up, including a selection of endotracheal tubes, a low resistance breathing system, and monitoring equipment suitable for Max’s small size.
Anaesthetic Plan
Nicki was confident that Max had been sufficiently stabilised in terms of hydration status, and premedicated Max with some intravenous methadone, the first of several analgesic drugs creating a multi-modal analgesia approach for his abdominal surgery. Gut motility is governed by the parasympathetic nervous system, and so if an animal is left in pain, the stimulation of the sympathetic nervous system will downregulate the parasympathetic nervous system, leading to potential gut ileus. A lidocaine continuous rate infusion was also included in the analgesic plan; in addition to producing analgesia, lidocaine when given intravenously is also pro-kinetic, anti-endotoxic and anti-inflammatory, all potentially beneficial effects for this puppy’s condition. Nicki was careful to use the lower end of the infusion rate range, due to the immature liver metabolism. Micro doses of ketamine given intramuscularly just before the start of surgery and just prior to abdominal closure provided additional somatic analgesia. Intravenous paracetamol was administered instead of a typical non-steroidal anti-inflammatory drug (NSAIDs) due to the use of NSAIDs being contraindicated in animals with gastro-intestinal disease.
Max appeared mildly sedated after his methadone pre-anaesthetic medication. Instead of increasing his level of sedation with an alpha 2 adrenergic agonist (as Nicki was avoiding the production of bradycardia – see cardiovascular system note above) or acepromazine (which has a long duration of action and may promote hypothermia), Nicki co-induced his anaesthetic with midazolam and alfaxalone intravenously. After pre-oxygenation using a mask (see the respiratory system comments above) endotracheal intubation was performed with a 5mm PVC tube. Max’s anaesthetic was maintained with isoflurane in oxygen, delivered via a Mapelson D T-piece. Fluid therapy with Hartmann’s solution was continued throughout the anaesthetic.
Monitoring included capnography, pulse oximetry, oesophageal temperature and indirect blood pressure. Both active and passive methods of warming were employed during surgical preparation and the surgical procedure; a minimally invasive midline coeliotomy with jejunal enterotomy to remove the dummy teat. As the radiographic and ultrasonic finding corroborated the palpation of a single foreign body, a 2cm long incision was made, just enough to allow exteriorisation of the affected loop of small intestine. Max’s anaesthetic was stable; there was a transient response to closure of the abdominal wall which was treated with a low dose of intravenous fentanyl. The team’s efforts at hypothermia prevention paid off; Max moved into the recovery area with an oesophageal temperature of 37.5°C, certainly helped by the short duration of surgery (just 25 minutes!) and minimal exposure of the abdominal cavity.

Outcome
Max was hospitalised overnight, encouraged into early enteral feeding, and maintained with intravenous fluid therapy. Analgesia (methadone IV, lidocaine CRI and paracetamol IV) was continued albeit either at the lower end of the CRI dose range (lidocaine), a reduced frequency (paracetamol) or in response to pain scores (methadone) to reflect Max’s immature biotransformation of drugs. Max recovered from anaesthesia and surgery well, and was discharged home the next day.
If you are presented with a case that requires anaesthesia or sedation, and it feels a little out of your comfort zone, contact Dr Nicki Grint at Virtual Veterinary Specialists to talk through the case. Nicki will set out the considerations and then work with you (taking into account the facilities and drugs that your clinic has), as well as the animal’s individual history and clinical findings to create a bespoke plan for the patient.
Acknowledgements
The author would like to thank Wallasey Vets4pets for permission to reproduce the radiograph, and Emma Hasleton (surgeon), Pablo Menendez Alegria (radiologist) and the team at Chestergates Veterinary Specialists for their expertise.
