A case of feline hypertension – more than meets the eye?
Dr Sarah Spencer
VVS Small Animal Internal Medicine Specialist

Do you offer senior health consultations in your practice?
VVS Internal Medicine Specialist Dr Sarah Spencer brings you an interesting and though-provoking case study. Read about Mimi, who first presented at her routine senior health consultation.
This is a case of Mimi, an 11-year-old domestic shorthair who presented for a routine senior health check-up. The owner had no concerns and physical exam was unremarkable. There had been a 200g weight loss since her last visit six months ago (weight 3.17 kg; body condition score 5/9; muscle condition score 2/3).
As part of the standard ‘senior cat’ consultation, blood pressure was measured using Doppler sphygmomanometer. This was prior to physical examination, once Mimi had settled and whilst the history was being obtained. The owner was present and assisted in comforting Mimi but she remained anxious and intermittently growling during measurement. Five systolic blood pressure (SBP) measurements were obtained, giving an average reading of 164 mmHg.
According to ACVIM guidelines 1, SBP ≥ 160 mmHg is considered hypertensive, with a moderate risk of target organ damage. However, Mimi was obviously stressed during measurement, and some element of “white coat” or “situational” hypertension was considered likely. To better understand the blood pressure status, retinal examination was performed to assess for retinopathy or choroidopathy. Mimi’s retinal vessels were slightly torturous, and there were a couple of tiny pink spots on the left retina (Fig.1 and Fig. 2).


Fig.1 and Fig. 2 – Mimi’s retinal examination
Blood tests were taken for a routine senior panel, including total T4 and SDMA. Urine collection via cystocentesis was not possible due to inadequate bladder size.
This showed a mild hypokalaemia and subsequent increase in sodium/potassium ratio. Otherwise blood results were within normal limits.
| Test | Result | Alert | Units | Reference Range |
| Potassium | 3.39 | Low | mmol/L | 3.50-5.50 |
| Sodium | 155 | mmol/L | 145.0-157.0 | |
| Sodium/potassium ratio | 45.72 | High | mmol/L | 28.00-40.00 |
Differential diagnoses considered for hypokalaemia were as followed:
- Increased loss
- Gastrointestinal losses
- E.g. vomiting, diarrhoea
- E.g. body cavity effusions, gastrointestinal tract distension
- Renal losses
- Aldosterone-driven
- Primary hyperaldosteronism (adrenal neoplasia or hyperplasia)
- Secondary hyperaldosteronism (e.g. renal disease)
- RAAS stimulation in response to hypovolaemia/hypotension
- Polyuria or diuresis (causes increased distal tubular flow rate and enhanced potassium excretion)
- Renal tubular disease
- E.g. chronic kidney disease (CKD), proximal renal tubular acidosis
- Loop diuretics
- Aldosterone-driven
- Gastrointestinal losses
- Decreased intake
- Hyporexia/anorexia. N.B. Decreased intake in small animals rarely results in hypokalemia unless there are additional potassium losses
- Low potassium diet
- Transcellular shifts
- From extra- to intercellular fluid (in exchange for hydrogen ions) due to primary respiratory or primary metabolic alkalosis causing alkalemia, insulin release/administration, catecholamine release
- Endotoxaemia (endotoxins stimulate the Na/K ATPase pump in muscle cells and promote insulin release)
- Artefact
- Severe lipemia
- Hyperglobulinemia (due to immunoglobulins)
Decreased intake, transcellular shifts and artefact could be largely ruled out given Mimi’s history and other biochemical results. Increased potassium loss was therefore considered most likely, with renal losses highest given the lack of gastrointestinal signs and without evidence of body cavity effusions. To further assess her renal function, the owner was asked to provide a home collected urine sample, but this did not happen. Indeed, given that hypokalaemia was mild and Mimi was very well in herself, no further action was not taken at this stage.
Interestingly, hypokalaemia has been associated with hypertension, hypertensive retinopathy, and chronic kidney disease in cats2–4. Regarding Mimi’s blood pressure, it was decided to recheck it in within eight weeks, as per ACVIM guidelines1, given that the retinal changes were deemed equivocal and no underlying condition as a cause for hypertension had been detected thus far.
In fact, Mimi did not return for reassessment until six months later. Her weight, body condition and physical examination were unchanged. Repeat SBP measurement (using the same size cuff and forelimb location) showed severe hypertension, with an average reading of 196 mmHg. Retinal examination showed bilateral multifocal hyporeflective areas of oedema, focal haemorrhages and areas of partial retinal detachment. Thus Mimi could be definitively diagnosed as hypertensive, with a current severe risk of target organ damage.

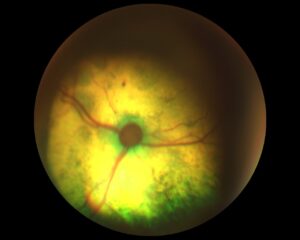
Fig. 3 and Fig. 4 – Mimi’s retinal re-examination
Repeat serum biochemistry and TT4 measurement were performed, particularly to reassess potassium and renal parameters. Serum potassium was persistently reduced but stable at 3.35 mmol/L; renal parameters remained within normal limits. Given that increasing serum creatinine concentrations within reference range may indicate declining kidney function, this was also scrutinised but not found.
Mimi was started on amlodipine besylate (Amodip®) due to its well-established efficacy in cats. A 0.625mg SID dose was initiated but future dose escalation was considered likely, given that SBP was close to >200 mmHg and data has shown an increase likelihood for higher dose amlodipine in such cases.5 At the time, telmisartan (Semintra®) was not licensed for use in feline hypertension, and to date studies directly comparing the efficacy of telmisartan and amlodipine are lacking. Furthermore, the safety and efficacy of telmisartan for the management of systemic hypertension above 200 mmHg has not been investigated. It was stressed to Mimi’s owner that repeat blood pressure measurement was needed within a week of starting therapy to document sufficient antihypertensive effect. Re-examination documented a SBP of 146 mmHg and 0.625 mg amlodipine SID was continued.
Follow-up was performed four months later. Mimi was reported to be mildly polydipsic, and she had lost further weight and body condition (weight 3.01 kg, BCS 4/9, MCS 1/3). On abdominal palpation, a mid/dorsal abdominal mass approximately the size of a peach stone was appreciated. SBP averaged 142 mmHg. Complete blood count was normal; repeat serum biochemistry showed:
| Test | Result | Alert | Units | Reference Range |
| SDMA | 18 | High | mmol/L | 1-14 |
| Creatinine | 109.0 | umol/L | 80.0-203.0 | |
| Potassium | 2.42 | Low | mmol/l | 3.50-5.50 |
| Sodium | 156 | mmol/l | 145.0-157.0 | |
| Sodium/potassium ratio | 64.46 | High | mmol/l | 28.00-40.00 |
Urine was obtained by cystocentesis and reduced concentrating ability was documented (USG 1.018). This in combination with increased SDMA indicated likely reduced kidney function. Findings were consistent with CKD IRIS stage 2, although persistence of these changes would need to be documented before definitively diagnosing CKD.
Mimi’s problem list at this stage:
- Mid abdominal mass
- Hypertension (currently controlled)
- Moderate hypokalaemia
- Likely reduced renal function based on isosthenuria and increased SDMA
- Polyuria
- Weight loss; reducing BCS and MCS
Based on these problems, primary hyperaldosteronism (PA) due to an adrenal gland tumour was strongly suspected. Abdominal imaging (ultrasound or CT scan) and plasma aldosterone concentration (PAC) measurement was offered to the owner. They elected for the latter only, as they did not want Mimi to undergo more intensive investigations given that they would not pursue surgical adrenalectomy if the diagnosis was confirmed. Potassium gluconate was started (one Chemeyes-K® 468 mg capsules BID) pending results.
PAC was confirmed to be markedly elevated (4605 pmol/L, reference range 194-388). A presumed diagnosis of PA was made. Spironolactone (Prilactone®) was initiated at 2mkg/kg SID. Repeat renal biochemistry profile one week later showed potassium levels to have normalised (3.8 mmol/L); SBP had reduced somewhat to 130 mmHg.
Mimi was re-examined two months later and her abdominal mass had increased in size. She was intermittently hyporexic and with diarrhoea. SBP, serum potassium and creatinine were stable. Shortly after this visit her owner elected to euthanase Mimi due to reduced quality of life. Permission was obtained for gross post-mortem examination and an adrenal tumour was confirmed.

Reflection on this case
For me, this case is memorable due to the mild and fairly innocuous initial indicators of hyperaldosteronism. The classic presentations in feline ‘Conn’s syndrome’ are described as a weak cat with cervical ventroflexion and a plantigrade stance, or the triad of hypertension, CKD and hypokalaemia on initial presentation. This is often not the case and clinical signs can be more subtle and insidious. This case also highlights the importance of close monitoring of feline hypertensives and following up on borderline clinicopathological changes.
Dose escalation of amlodipine (to 1.25 mg/cat) at the first recheck visit when Mimi’s SBP was 146 mmHg may have been advisable. Although the optimum level of blood pressure control is unknown in hypertensive cats, ACVIM guidelines1 recommend aiming for <140 mmHg, and a study showed improved survival in cats that had an amlodipine dose change (presumably as a proxy measure of BP control and diligent case monitoring).6 Urine protein/creatinine ratio should be measured in hypertensive cats on therapy as proteinuria is a key predictor of survival7, yet less than 10% hypertensive cats in primary practice underwent UPC measurement in one study6. Should proteinuria (UPC >0.4) be documented, telmisartan may be advantageous as an adjunct therapy for its antiproteinuric and RAAS-blocking effect.
Points of interest regarding feline primary hyperaldosteronism
In primary mineralocorticoid excess, plasma aldosterone concentration (PAC) is high and plasma renin activity (PRA) is low. However PRA measurement is rarely available and reference ranges vary. PAC was markedly increased in Mimi but interpretation of PAC can be challenging in some cases, overlapping with levels more consistently seen in secondary hyperaldosteronism due to CKD and/or hypertension8. Even moderately increased PAC is inappropriate in the presence of hypokalaemia. Urine aldosterone/cortisol ratio and use of a fludrocortisone suppression test are sometimes mentioned in the diagnosis of PA but shouldn’t be relied on due to a paucity of evidence. Recently, an oral telmisartan suppression test was evaluated as a diagnostic test for feline PA but was not able to distinguish reliably between PA and healthy middle-aged cats or cats with diseases that may result in secondary hyperaldosteronism.9 Idiopathic micronodular adrenal hyperplasia is also described as a cause for hyperaldosteronism in cats but this condition and its pathogenesis is poorly understood.10 PAC is usually only slightly elevated or within the upper limit of the reference range in this condition.
Most feline mineralocorticoid-producing adrenal tumours are malignant. Medical treatment may lead to resolution of clinical signs but complete normalisation of potassium levels in particular may not be achieved. Importantly, amlodipine is often required as the antihypertensive effect of spironolactone alone may not be sufficient. After complete excision of a unilateral non-metastasised mass, the prognosis is generally excellent and long-term medication may not be required.
Feline medicine cases can be challenging to manage in house, especially when they don’t present as you would expect from the text book!
If you would like to discuss a clinical case with Dr Sarah Spencer or any of the VVS Internal Medicine team get in touch by clicking the link below or email the VVS team at [email protected].
References / further reading
1. Acierno MJ, Brown S, Coleman AE, Jepson RE, Papich M, Stepien RL, et al. ACVIM consensus statement: Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med. 2018;32(6):1803–22.
2. Syme HM, Barber PJ, Markwell PJ, Elliott J. Prevalence of systolic hypertension in cats with chronic renal failure at initial evaluation. J Am Vet Med Assoc. 2002;220(12):1799–804.
3. Sansom J, Rogers K, Wood JL. Blood pressure assessment in healthy cats and cats with hypertensive retinopathy. Am J Vet Res. 2004;65:245–252.
4. Elliott J, Syme HM, Markwell PJ. Acid‐base balance of cats with chronic renal failure: effect of deterioration in renal function. J Small Anim Pract. 2003;44:261–8.
5. Bijsmans ES, Doig M, Jepson RE, Syme HM, Elliott J, Pelligand L. Factors influencing the relationship between the dose of amlodipine required for blood pressure control and change in blood pressure in hypertensive cats. J Vet Intern Med. 2016;30(5):1630–6.
6. Conroy M, Chang YM, Brodbelt D, Elliott J. Survival after diagnosis of hypertension in cats attending primary care practice in the United Kingdom. J Vet Intern Med. 2018;32(6):1846–55.
7. Jepson RE, Elliott J, Brodbelt D, Syme HM. Effect of control of systolic blood pressure on survival in cats with systemic hypertension. J Vet Intern Med. 2007;21(3):402–9.
8. Javadi S, Djajadiningrat-Laanen SC, Kooistraa HS, van Dongen AM, Voorhout G, van Sluijs FJ, et al. Primary hyperaldosteronism, a mediator of progressive renal disease in cats. Domest Anim Endocrinol. 2005;28:85–104.
9. Kurtz M, Fabrès V, Dumont R, Chetboul V, Chahory S, Saponaro V, et al. Prospective evaluation of a telmisartan suppression test as a diagnostic tool for primary hyperaldosteronism in cats. J Vet Intern Med. 2023 May 29. doi: 10.1111/jvim.16741. Epub ahead of print. PMID: 37246725.
10. Kooistra HS. Primary Hyperaldosteronism in Cats: An Underdiagnosed Disorder. Vol. 50, Veterinary Clinics of North America – Small Animal Practice. 2020.
